Bcs Class I
Biopharmaceutics Classification System (BCS) are intended only to investigate. Therefore, both BCS Class I (high solubility and high permeability) and Class.
 Views
ViewsBiopharmaceutical Classification System (BCS) is a scientific framework for classifying drug substances based on their aqueous solubility and intestinal permeability
when the dissolution rate is much greater than the gastric emptying, dissolution is not likely to be rate-limiting
the pharmaceutical industry

The Biopharmaceutics Classification System is a system to differentiate the drugs on the basis of their solubility and permeability.[1]
This system restricts the prediction using the parameters solubility and intestinal permeability. The solubility classification is based on a United States Pharmacopoeia (USP) aperture. The intestinal permeability classification is based on a comparison to the intravenous injection. All those factors are highly important because 85% of the most sold drugs in the United States and Europe are orally administered[citation needed].
BCS classes[edit]
According to the Biopharmaceutical Classification System (BCS) drug substances are classified to four classes upon their solubility and permeability:[1]Jab tak hai jaan online.
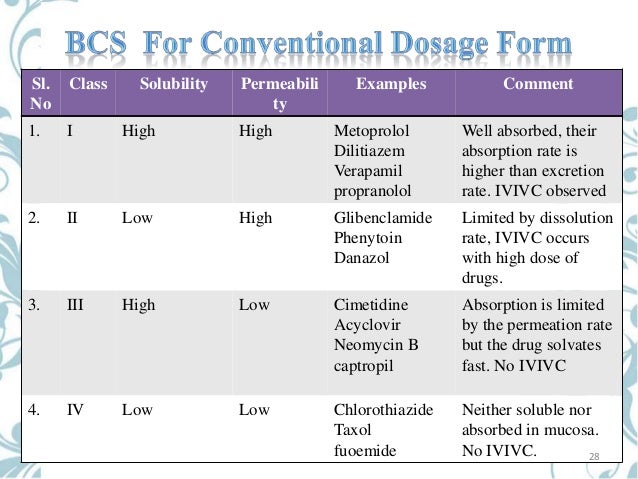
- Class I - high permeability, high solubility
- Example: metoprolol, paracetamol[2]
- Those compounds are well absorbed and their absorption rate is usually higher than excretion.
- Class II - high permeability, low solubility
- Example: glibenclamide, bicalutamide, ezetimibe, aceclofenac
- The bioavailability of those products is limited by their solvation rate. A correlation between the in vivo bioavailability and the in vitro solvation can be found.
- Class III - low permeability, high solubility
- Example: cimetidine
- The absorption is limited by the permeation rate but the drug is solvated very fast. If the formulation does not change the permeability or gastro-intestinal duration time, then class I criteria can be applied.
- Class IV - low permeability, low solubility
- Example: Bifonazole
- Those compounds have a poor bioavailability. Usually they are not well absorbed over the intestinal mucosa and a high variability is expected.
Definitions[edit]
The drugs are classified in BCS on the basis of solubility, permeability, and dissolution.
Solubility class boundaries are based on the highest dose strength of an immediate release product. A drug is considered highly soluble when the highest dose strength is soluble in 250 ml or less of aqueous media over the pH range of 1 to 7.5. The volume estimate of 250 ml is derived from typical bioequivalence study protocols that prescribe administration of a drug product to fasting human volunteers with a glass of water.
Permeability class boundaries are based indirectly on the extent of absorption of a drug substance in humans and directly on the measurement of rates of mass transfer across human intestinal membrane. Alternatively non-human systems capable of predicting drug absorption in humans can be used (such as in-vitro culture methods). A drug substance is considered highly permeable when the extent of absorption in humans is determined to be 90% or more of the administered dose based on a mass-balance determination or in comparison to an intravenous dose.
For dissolution class boundaries, an immediate release product is considered rapidly dissolving when no less than 85% of the labeled amount of the drug substance dissolves within 15 minutes using USP Dissolution Apparatus 1 at 100 RPM or Apparatus 2 at 50 RPM in a volume of 900 ml or less in the following media: 0.1 N HCl or simulated gastric fluid or pH 4.5 buffer and pH 6.8 buffer or simulated intestinal fluid.
See also[edit]
Bcs Class Ii Drugs
- ADME
References[edit]
- ^ abMehta M (2016). Biopharmaceutics Classification System (BCS): Development, Implementation, and Growth. Wiley. ISBN978-1-118-47661-1.
- ^https://www.ema.europa.eu/documents/scientific-guideline/draft-paracetamol-oral-use-immediate-release-formulations-product-specific-bioequivalence-guidance_en.pdf
Further reading[edit]
- Folkers G, van de Waterbeemd H, Lennernäs H, Artursson P, Mannhold R, Kubinyi H (2003). Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability (Methods and Principles in Medicinal Chemistry). Weinheim: Wiley-VCH. ISBN3-527-30438-X.
- Amidon GL, Lennernäs H, Shah VP, Crison JR (March 1995). 'A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability'. Pharm. Res. 12 (3): 413–20. PMID7617530.
External links[edit]
- BCS guidance of the U.S. Food and Drug Administration