Schedule 2 Prescription Drugs
This is the list of Schedule II drugs as defined by the United StatesControlled Substances Act.[1]The following findings are required for drugs to be placed in this schedule:[2]
Multiple Prescriptions of Schedule II Drugs Drug Enforcement Administration regulations allow practitioners to provide individual patients with multiple prescriptions, to be filled sequentially, for the same Schedule II controlled substance, with such multiple prescriptions having the combined effect of allowing a patient to receive over time up to a 90-day supply of that controlled substance. The prescription regarding (B) and (C) above. (2) A written prescription for a controlled dangerous substance in Schedule II becomes invalid thirty (30) days after the date of issuance, with day one (1) of the thirty (30) day period being the first day after date of issuance.
- The drug or other substance has a high potential for abuse.
- The drug or other substance has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions.
- Abuse of the drug or other substances may lead to severe psychological or physical dependence.
The complete list of Schedule II drugs follows.[1] The Administrative Controlled Substances Code Number for each drug is included.
| ACSCN | Class | Drug |
|---|---|---|
| 9050 | opiate | Codeine |
| 9334 | opiate | Dihydroetorphine |
| 9190 | opiate | Ethylmorphine |
| 9059 | opiate | Etorphine hydrochloride |
| 9640 | opiate | Granulated opium |
| 9193 | opiate | Hydrocodone |
| 9150 | opiate | Hydromorphone |
| 9260 | opiate | Metopon |
| 9300 | opiate | Morphine |
| 9610 | opiate | Opium extracts |
| 9620 | opiate | Opium fluid |
| 9330 | opiate | Oripavine |
| 9143 | opiate | Oxycodone |
| 9652 | opiate | Oxymorphone |
| 9639 | opiate | Powdered opium |
| 9600 | opiate | Raw opium |
| 9333 | opiate | Thebaine |
| 9630 | opiate | Tincture of opium |
| opiate | Opium poppy and poppy straw | |
| 9040 | stimulant | Coca, leaves and any salt, compound, derivative or preparation of coca leaves |
| 9041 | stimulant | Cocaine, and its salts, isomers, derivatives and salts of isomers and derivatives |
| 9180 | stimulant | Ecgonine, and its salts, isomers, derivatives and salts of isomers and derivatives |
| 9670 | opiate | Concentrate of poppy straw (the crude extract of poppy straw in either liquid, solid or powder form which contains the phenanthrene alkaloids of the opium poppy) |
| 9737 | opioid | Alfentanil |
| 9010 | opiate | Alphaprodine |
| 9020 | opioid | Anileridine |
| 9800 | opiate | Bezitramide |
| 9273 | opioid | Bulk dextropropoxyphene (non-dosage forms) |
| 9743 | opioid | Carfentanil |
| 9120 | opiate | Dihydrocodeine |
| 9170 | opioid | Diphenoxylate |
| 9801 | opioid | Fentanyl |
| 9226 | opioid | Isomethadone |
| 9648 | opiate | Levo-alphacetylmethadol |
| 9210 | opiate | Levomethorphan |
| 9220 | opiate | Levorphanol |
| 9240 | opioid | Metazocine |
| 9250 | opioid | Methadone |
| 9254 | opiate intermediate | Methadone intermediate: 4-cyano-2-dimethylamino-4,4-diphenyl butane |
| 9802 | opiate intermediate | Moramide intermediate: 2-methyl-3-morpholino-1,1-diphenylpropane-carboxylic acid |
| 9230 | opioid | Pethidine (meperidine) |
| 9232 | opiate intermediate | Pethidine intermediate A: 4-cyano-1-methyl-4-phenylpiperidine |
| 9233 | opiate intermediate | Pethidine intermediate B, ethyl-4-phenylpiperidine-4-carboxylate |
| 9234 | opiate intermediate | Pethidine intermediate C, 1-methyl-4-phenylpiperidine-4-carboxylic acid |
| 9715 | opiate | Phenazocine |
| 9730 | opiate | Piminodine |
| 9732 | opiate | Racemethorphan |
| 9733 | opiate | Racemorphan |
| 9739 | opiate | Remifentanil |
| 9740 | opiate | Sufentanil |
| 9780 | opiate | Tapentadol |
| 1100 | stimulant | Amphetamine, its salts, optical isomers, and salts of its optical isomers (Adderall) |
| 1105 | stimulant | Methamphetamine, its salts, isomers, and salts of its isomers |
| 1631 | stimulant | Phenmetrazine and its salts |
| 1724 | stimulant | Methylphenidate (Ritalin, Concerta, etc.) |
| 1205 | stimulant | Lisdexamfetamine (Vyvanse), its salts, isomers, and salts of its isomers |
| 2125 | depressant | Amobarbital |
| 2550 | depressant | Glutethimide |
| 2270 | depressant | Pentobarbital |
| 7471 | depressant | Phencyclidine |
| 2315 | depressant | Secobarbital |
| 7379 | hallucinogen | Nabilone |
| 8501 | precursor | Phenylacetone |
| 7460 | precursor | 1-phenylcyclohexylamine |
| 8603 | precursor | 1-piperidinocyclohexanecarbonitrile (PCC) |
| 8333 | precursor | 4-anilino-N-phenethyl-4-piperidine (ANPP) |
References[edit]
- ^ ab21 CFR1308.12 (CSA Sched II) with changes through 77 FR64032 (Oct 18, 2012). Retrieved September 6, 2013.
- ^21 U.S.C.§ 812(b)(4) retrieved October 7, 2007
Schedule 1 Prescription Drugs
This second article of a 4-part series on key components of the Federal Controlled Substances Act will discuss the requirements for controlled substances prescriptions. For a prescription for a controlled substance to be considered valid, it must be “issued for a legitimate medical purpose by a registered practitioner acting in the usual course of sound professional practice.”1Registered practitioner refers to any health care professional who is authorized to prescribe controlled substances within the area in which he or she is licensed to practice and who is registered with the Drug Enforcement Agency (DEA) or is exempt from registration.2 All of the following must be included in a prescription for a controlled substance1:
Issue date
Name and address of patient
Name, address, and DEA registration number of practitioner
Drug name
Strength of drug
Dosage form (ie, tablet, suspension, etc)
Quantity prescribed
Directions for use
Refills (if authorized)
Manual signature of the prescriber
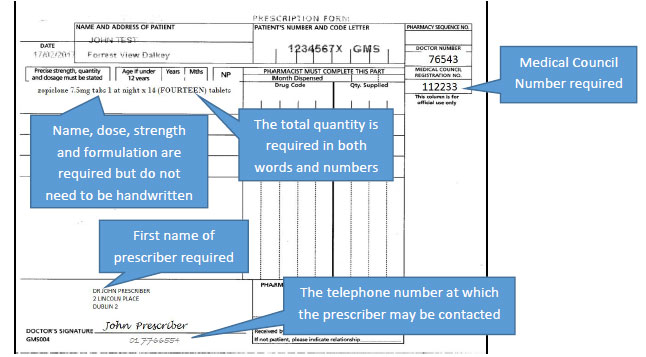
Schedule II prescriptions must be presented to the pharmacy in written form and signed by the prescriber.1 There are no federal quantity limits on Schedule II prescriptions.2 In addition, there is no federal time limit on when a Schedule II prescription must be filled after being signed by a prescriber. That being said, the pharmacist must ensure that the controlled substance is being prescribed for a legitimate medical purpose; the quantity of the medication prescribed and the time between signing and filling of a prescription may play a role in this decision. Note that state laws may have stricter rules.
A prescription for a Schedule II medication may be phoned into the pharmacy in an emergency situation.1 The prescriber must follow-up the phone prescription with a written prescription to the pharmacy within 7 days. Faxed Schedule II prescriptions are generally permitted, however, the pharmacist must receive the original, signed written prescription before dispensing the Schedule II controlled substance to the patient.2 There are 3 scenarios in which a facsimile Schedule II prescription may serve as an original written prescription. These include the following:
The health care provider is prescribing a Schedule II narcotic to be compounded for direct administration to a patient by intravenous, intramuscular, subcutaneous, or intraspinal infusion.
The provider is prescribing Schedule II medications to patients within a long-term care facility, which are normally filled and delivered to the patients within the facility by the pharmacy. Nba 2k17 download.
The provider is prescribing Schedule II medications to a patient in hospice care as certified by Medicare or licensed by the state.
Prescriptions for Schedules III to V controlled substances may be written, orally communicated, or faxed to the pharmacy.1
Not all prescriptions for controlled substances can be refilled.1 Schedule II medications may not be refilled; a new prescription must be written every time. Medications classified as Schedule III or IV controlled substances may be refilled up to 5 times in a 6-month period. Schedule V medications may be refilled as authorized by the prescriber. For refills of any controlled substance, the dispensing pharmacist’s initials, date of refill, and amount dispensed must be written on the back of the prescription.2
One mechanism to verify the validity of a controlled substance prescription is through the DEA registration number provided by the practitioner.2 DEA registration numbers contain 2 letters followed by a computer-generated sequence of 7 numbers. The first letter in the DEA registration is generally an A, B, or M. Prior to October 1, 1985, DEA registration numbers began with the letter A. Registration numbers issued after this date start with the letter B. Mid-level practitioners, such as advanced nurse practitioners and physician assistants, have registration numbers beginning with the letter M. The second letter in the registration number is the first letter of the practitioner’s last name (ie, J for Jackson or W for White). The computer-generated sequence of numbers can be verified using the following formula: add the sum of the first, third, and fifth digits to twice the sum of the second, fourth, and sixth digits. The total should be a number whose last digit is the same as the last digit of the DEA number on the prescription.
Health care providers with prescribing authority, when acting within the usual course of business at a hospital or other health care institution, may prescribe controlled substances under the DEA registration number of the hospital or institution.2 Examples of practitioners who may use a hospital’s DEA registration number include physician interns and residents as well as medical house staff or mid-level practitioners such as physician assistants or advanced nurse practitioners. The hospital or other institution must authorize the health care provider to prescribe under its registration number. A specific internal code number must be assigned to each authorized practitioner.
The health care institution must keep an up-to-date list of all internal codes with the corresponding practitioner.2 If the pharmacy has any doubt regarding a controlled substance prescription from a provider using a health care institution’s DEA number, the pharmacist may contact the institution to verify the legitimacy of the prescription. As mentioned previously, mid-level practitioners such as nurse midwives, nurse practitioners, nurse anesthetists, clinical nurse specialists, physician assistants, and optometrists may be granted DEA registration numbers and may prescribe controlled substances. However, registration is contingent upon authority granted by the state in which they are licensed. Pharmacists must be familiar with the controlled substances act in their state to determine which health care providers may or may not prescribe any controlled substances and, if so, which schedules may be prescribed.
On December 19, 2007, a DEA regulation came into effect that allows a prescriber to issue multiple prescriptions authorizing an individual patient to receive a total of up to a 90-day supply of a Schedule II controlled substance.2 However, this is allowable only under the following conditions:
Every Schedule II prescription must be written on a separate prescription blank.
Each Schedule II prescription must be written for a legitimate medical purpose by an authorized prescriber during the usual course of professional practice.
The prescriber must indicate on each prescription the earliest date on which the prescription can be filled by the pharmacy; an exception to this rule would be for the first prescription if the prescriber intends for that prescription to be filled immediately.
The prescriber must determine that providing multiple Schedule II prescriptions to the patient does not increase the risk of diversion or abuse.
State law must allow for the issuance of multiple prescriptions.
The individual prescriber must comply fully with all other applicable requirements under the Controlled Substance Act and the Code of Federal Regulations, as well as any additional requirements under state law.
The third RxLegal column in this series will discuss ordering and record-keeping requirements for controlled substances.
REFERENCES
